Understanding Med-PaLM: Google’s Generative AI in Healthcare
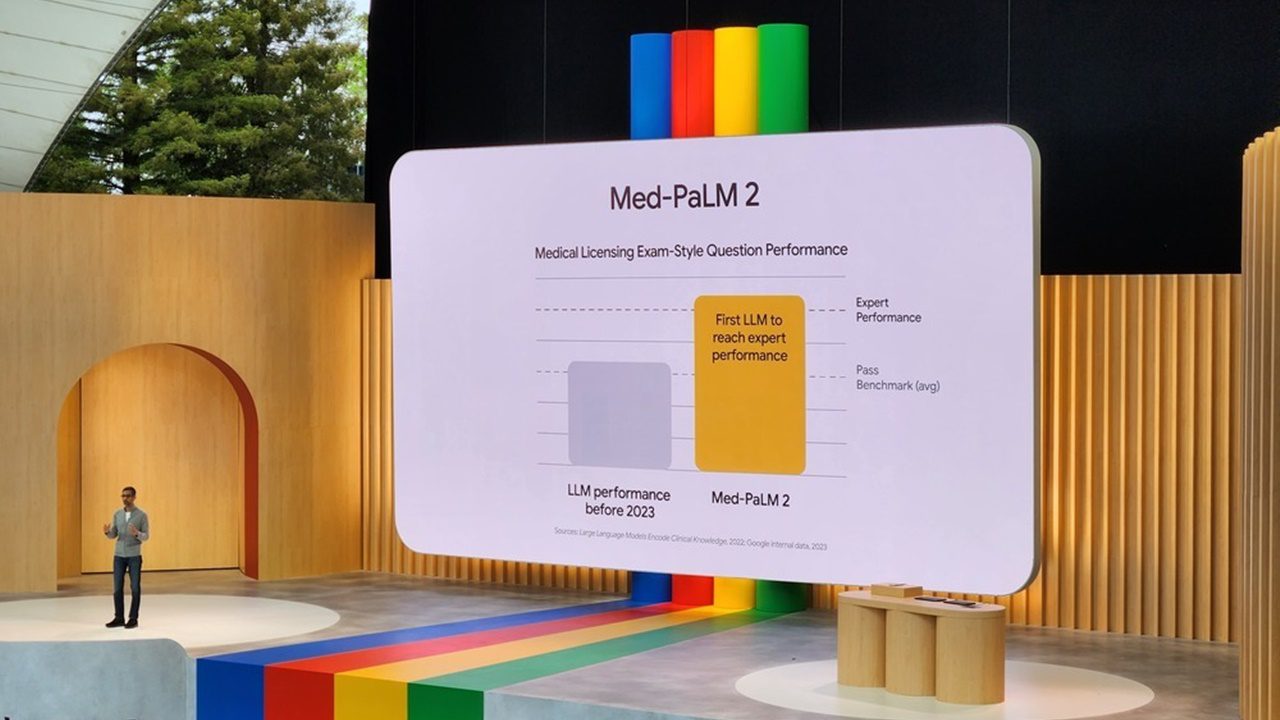
Med-PaLM, a groundbreaking large language model (LLM) developed by Google, signifies a significant advancement in the application of artificial intelligence (AI) within the healthcare sector. Trained specifically with a vast corpus of medical data, Med-PaLM aims to revolutionize the way medical professionals access and interpret